what is the electron configuration for magnesium|zr4+ electron configuration : Bacolod Electron configuration of magnesium. The Electron configuration of magnesium is [Ne] 3s 2. The chemical element magnesium is distinguished by its atomic number 12, its . A essência do jogo Aviãozinho Bet. O Aviator é um jogo de acidente multiplayer, no qual você controla um aviãozinho. No Aviator, você pode obter lucro através do aumento das apostas e multiplicadores, que variam de 0 a 100, .
PH0 · zr4+ electron configuration
PH1 · potassium full electron configuration
PH2 · electron configuration of v3+ ion
PH3 · electron configuration of all elements
PH4 · electron configuration chart
PH5 · abbreviated electron configuration chart
PH6 · Iba pa
If you have landed here searching for EDV 2025 Result, then you have arrived in right place. The US Diversity Visa 2025 Result is going to be announced today i.e 4th May 2024 Saturday (21:45 Nepal Time). dvprogram.state.gov is the official website to check your dv result 2025. Contents hide 1 How to Check Your .
Electron configurationThe arrangements of electrons above the last (closed shell) noble gas. Melting pointThe temperature at which the solid–liquid phase change occurs. Boiling . Magnesium Electron Configuration: Mg is a chemical element that has the symbol Mg. The atomic number of Magnesium is 12. It is a grey shiny solid that bears a close physical resemblance to five other .A step-by-step description of how to write the electron configuration for Magnesium (Mg). In order to write the Mg electron configuration we first need to k.
Electron configuration of Magnesium is [Ne] 3s2. Possible oxidation states are +2. Magnesium occurs naturally only in combination with other elements, where it invariably .
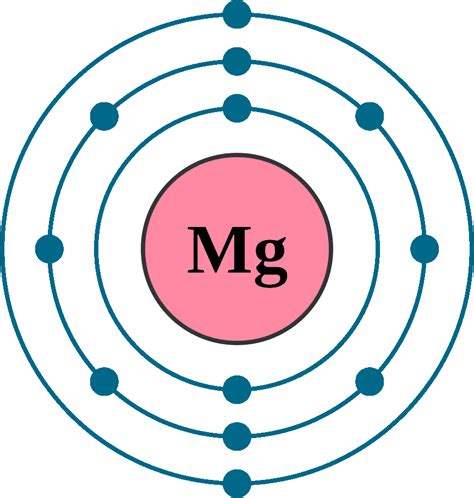
Figure \(\PageIndex{2}\): Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are .
Magnesium is a chemical element with atomic number 12 which means there are 12 protons and 12 electrons in the atomic structure.The chemical symbol for Magnesium is Mg. Electron Configuration and Oxidation States of Magnesium. Electron configuration of Magnesium is [Ne] 3s2. Possible oxidation states are +2. . Magnesium (Mg) Orbital diagram, Electron configuration, and Valence electrons. Magnesium has an atomic number of 12 belongs to Group 2 also known as the alkaline earth metals. It is situated in the S-block of the periodic table. Magnesium has the symbol Mg and “it is the eighth-most abundant element in the Earth’s crust”.2.4 Electron Configurations is shared under a CC BY-NC-SA 4.0 license and was authored, remixed, and/or curated by LibreTexts. The electron configuration of an atom is the representation of the arrangement of .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).A step-by-step description of how to write the electron configuration for Magnesium (Mg). In order to write the Mg electron configuration we first need to k. Electron configuration chart of all Elements is mentioned in the table below.The Shorthand electron configuration (or Noble gas configuration) as well as Full . Electron configuration of Magnesium (Mg) [Ne] 3s 2: 1s 2 2s 2 2p 6 3s 2: 2, 8, 2: 13: Electron configuration of Aluminum (Al) [Ne] 3s 2 3p 1: 1s 2 2s 2 2p 6 3s 2 3p 1: 2, 8, .Figure 2.4.2 2.4. 2: Electron diagram for magnesium. The electron arrangement also shows the number of valence electrons which is two for magnesium because there are two electrons in the n = 3 n = 3 energy level which is the highest occupied energy level for magnesium. This corresponds to the 2+ 2 + charge formed when magnesium forms an .The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = + 1 2 ).Electron Configurations are an organized means of documenting the placement of electrons based upon the energy levels and orbitals groupings of the periodic table. . What is the electronic configuration of magnesium? Question #3df7e. Question #32589. Question #2262f.Magnesium is a chemical element of the periodic table with chemical symbol Mg and atomic number 12 with an atomic weight of 24.304 u and is classed as a alkaline earth metal. . of the periodic table:they each have the same electron configuration in their outer electron shell producing a similar crystal structure. Magnesium is the ninth most . Explanation: Magnesium is element 12, so it has 12 protons and 12 electrons. The electrons fill the orbitals, reaching 12 when the 3s orbital is filled. Answer link. 1s^2 2s^2 2p^6 3s^2 Magnesium is element 12, so it has 12 protons and 12 electrons. The electrons fill the orbitals, reaching 12 when the 3s orbital is filled.For example, the last electron added in the electron configuration of magnesium is the second 3s-electron. Mg + would have a configuration of 1s 2 2s 2 2p 6 3s 1. For Mg 2+, the electron configuration would be .What is the electronic configuration of magnesium (24 12 M g)? . 2,8,7 Which among the given element can be beaten into sheets easily? View Solution. Q5. The electronic configuration of sodium atom is 2, 8, 1 and chlorine atom is 2, 8, 7. The atoms will form a bond by of electrons. View Solution. Solve.
The electron configuration for magnesium is 1s 2 2s 2 2p 6 3s 2 . Magnesium is in the third row and second column on the periodic table. This means its. See full answer below.
The electron configuration and the orbital diagram are: Following hydrogen is the noble gas helium, which has an atomic number of 2. The helium atom contains two protons and two electrons. The first electron has the same four quantum numbers as the hydrogen atom electron ( n = 1, l = 0, ml = 0, ms = +1 2 m s = + 1 2 ).what is the electron configuration for magnesium zr4+ electron configurationWhat is the electron configuration for a magnesium ion, Mg? 1 s2 2 s2 2 p6 3 s1. 1 s2 2 s2 2 p6 3 s2. 1 s2 2 s2 2 p6. 1 s2 2 s2 2 p6 3 s2 3 p1.The alkaline earth metal magnesium (atomic number 12), with its 12 electrons in a [Ne] . When their electron configurations are added to the table (Figure 6.29), we also see a periodic recurrence of similar electron configurations in the outer shells of these elements. Because they are in the outer shells of an atom, valence electrons play . In this video we will write the electron configuration for Mg 2+, the Magnesium ion. We’ll also look at why Magnesium forms a 2+ ion and how the electron con.
To write the orbital diagram for the Magnesium atom (Mg) first we need to write the electron configuration for just Mg. To do that we need to find the number.
When this happens, it's usually because the owner only shared it with a small group of people, changed who can see it or it's been deleted.
what is the electron configuration for magnesium|zr4+ electron configuration